Reversible mechanofluorochromism of aniline-terminated phenylene ethynylenes†
Abstract
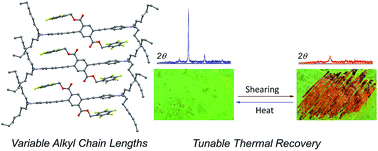
Seven three-ring phenylene-ethynylene (PE) structural analogs, differing only in the lengths of alkyl chains on terminal aniline substituents, show 50–62 nm bathochromic shifts in emission maxima in response to mechanical force (mechanofluorochromism, MC). These shifts are fully reversible with heat or solvent fuming. Shearing of these solids yields a transition from green-emitting crystalline phases to orange-emitting amorphous phases as established by differential scanning calorimetry and X-ray diffraction. Molecules with shorter alkyl chain lengths required higher temperatures to recover the hypsochromically shifted crystalline phases after grinding, while the recovery with chain lengths longer than butyl occurred at room temperature. In addition to this structure-dependent thermochromism, these compounds retain their MC properties in polymer hosts to various extents. The crystalline phases of these materials have PE chromophores that are twisted due to non-covalent perfluoroarene–arene (ArF–ArH) interactions involving perfluorophenyl pendants and the terminal rings of the PE chromophore, resulting in interrupted conjugation and an absence of chromophore aggregation. The MC behavior of an analog without the perfluoroarene rings is severely attenuated. This work demonstrates the general utility of twisted PEs as stimuli-responsive moieties and reveals clear structure–property relationships regarding the effects of alkyl chain length on these materials.


 Please wait while we load your content...
Please wait while we load your content...