Enantiocontrol by assembled attractive interactions in copper-catalyzed asymmetric direct alkynylation of α-ketoesters with terminal alkynes: OH⋯O/sp3-CH⋯O two-point hydrogen bonding combined with dispersive attractions†
Abstract
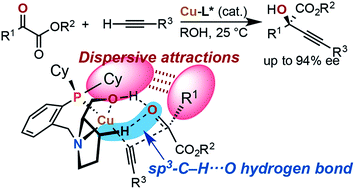
Copper-catalyzed asymmetric direct alkynylation of α-ketoesters with terminal alkynes with chiral prolinol–phosphine ligands, most preferably (αR,2S)-1-(2-dicyclohexylphosphinobenzyl)-α-neopentyl-2-pyrrolidinemethanol, afforded various enantioenriched chiral propargylic tertiary alcohols. Quantum-chemical calculations using the BP86 density functional including Grimme's empirical dispersion correction [DF-BP86-D3(BJ)-PCM(tBuOH)/TZVPP//DF-BP86-D3(BJ)/SVP] show the occurrence of OH⋯O/sp3-CH⋯O two-point hydrogen bonding between the chiral ligand and the carbonyl group of the ketoester in the stereo-determining transition states. Combined with the hydrogen-bonding interactions orienting the ketoester substrate, dispersive attractions between the chiral ligand (P-cyclohexyl groups) and the ketoester in the favored transition states, rather than steric repulsions in the disfavored transition state explain the enantioselectivity of the asymmetric copper catalysis.


 Please wait while we load your content...
Please wait while we load your content...