Direct and indirect hyperpolarisation of amines using parahydrogen†
Abstract
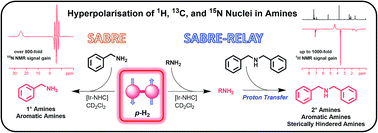
Nuclear Magnetic Resonance (NMR) and Magnetic Resonance Imaging (MRI) are two widely used techniques for the study of molecules and materials. Hyperpolarisation methods, such as Signal Amplification By Reversible Exchange (SABRE), turn typically weak magnetic resonance responses into strong signals. In this article we detail how it is possible to hyperpolarise the 1H, 13C and 15N nuclei of a range of amines. This involved showing how primary amines form stable but labile complexes of the type [Ir(H)2(IMes)(amine)3]Cl that allow parahydrogen to relay its latent polarisation into the amine. By optimising the temperature and parahydrogen pressure a 1000-fold per proton NH signal gain for deuterated benzylamine is achieved at 9.4 T. Additionally, we show that sterically hindered and electron poor amines that bind poorly to iridium can be hyperpolarised by either employing a co-ligand for complex stabilisation, or harnessing the fact that it is possible to exchange hyperpolarised protons between amines in a mixture, through the recently reported SABRE-RELAY method. These chemical refinements have significant potential to extend the classes of agent that can be hyperpolarised by readily accessible parahydrogen.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners


 Please wait while we load your content...
Please wait while we load your content...