Chiral probes for α1-AGP reporting by species-specific induced circularly polarised luminescence†
Abstract
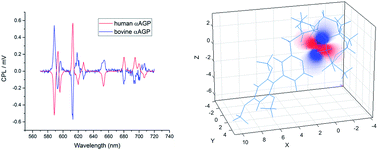
Luminescence spectroscopy has been used to monitor the selective and reversible binding of pH sensitive, macrocyclic lanthanide complexes, [LnL1], to the serum protein α1-AGP, whose concentration can vary significantly in response to inflammatory processes. On binding α1-AGP, a very strong induced circularly-polarised europium luminescence signal was observed that was of opposite sign for human and bovine variants of α1-AGP – reflecting the differences in the chiral environment of their drug-binding pockets. A mixture of [EuL1] and [TbL1] complexes allowed the ratiometric monitoring of α1-AGP levels in serum. Moreover, competitive displacement of [EuL1] from the protein by certain prescription drugs could be monitored, allowing the determination of drug binding constants. Reversible binding of the sulphonamide arm as a function of pH, led to a change of the coordination environment around the lanthanide ion, from twisted square antiprism (TSAP) to a square antiprismatic geometry (SAP), signalled by emission spectral changes and verified by detailed computations and the fitting of NMR pseudocontact shift data in the sulphonamide bound TSAP structure for the Dy and Eu examples. Such analyses allowed a full definition of the magnetic susceptibility tensor for [DyL1].


 Please wait while we load your content...
Please wait while we load your content...