Intramolecular hydride transfer onto arynes: redox-neutral and transition metal-free C(sp3)–H functionalization of amines†
Abstract
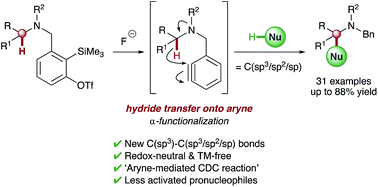
Transition metal-free intramolecular hydride transfer onto arynes is reported for the first time. This unique transformation is utilized in redox-neutral intermolecular α-functionalization reactions of different tertiary amines, generating C(sp3)–C(sp3/sp2/sp) bonds in a single synthetic operation. Deuterium labeling studies support initial cleavage of the α-C–H bond via intramolecular 1,5-hydride transfer onto the aryne, which leads to activation of a range of integrated pronucleophiles and ultimately affords a new approach to cross-dehydrogenative coupling reactions which utilize aryne intermediates.


 Please wait while we load your content...
Please wait while we load your content...