Formation of quaternary centres by copper catalysed asymmetric conjugate addition to β-substituted cyclopentenones with the aid of a quantitative structure–selectivity relationship†
Abstract
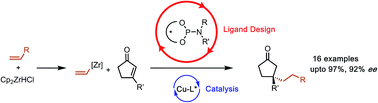
A new asymmetric conjugate addition method was developed for β-substituted cyclopentenones to form quaternary centres using alkylzirconocene nucleophiles giving up to 97% yield and 92% ee. Key to the reaction's success was the design of suitable phosphoramidite ligands which was aided by a linear quantitative structure–selectivity relationship (QSSR). QSSR models were created from the ligand screening data (a total of 36 ligands) which revealed important electronic and steric requirements and led to the synthesis of more enantioselective ligands. DFT calculations of competing transition structures enable the interpretation of the electronic and steric terms present in the QSSR models.


 Please wait while we load your content...
Please wait while we load your content...