Direct evidence of charge separation in a metal–organic framework: efficient and selective photocatalytic oxidative coupling of amines via charge and energy transfer†
Abstract
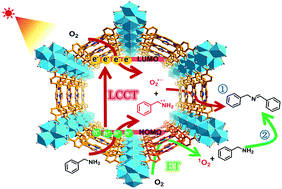
The selective aerobic oxidative coupling of amines under mild conditions is an important laboratory and commercial procedure yet a great challenge. In this work, a porphyrinic metal–organic framework, PCN-222, was employed to catalyze the reaction. Upon visible light irradiation, the semiconductor-like behavior of PCN-222 initiates charge separation, evidently generating oxygen-centered active sites in Zr-oxo clusters indicated by enhanced porphyrin π-cation radical signals. The photogenerated electrons and holes further activate oxygen and amines, respectively, to give the corresponding redox products, both of which have been detected for the first time. The porphyrin motifs generate singlet oxygen based on energy transfer to further promote the reaction. As a result, PCN-222 exhibits excellent photocatalytic activity, selectivity and recyclability, far superior to its organic counterpart, for the reaction under ambient conditions via combined energy and charge transfer.
- This article is part of the themed collection: In celebration of Chinese New Year


 Please wait while we load your content...
Please wait while we load your content...