Regioselective Simmons–Smith-type cyclopropanations of polyalkenes enabled by transition metal catalysis†
Abstract
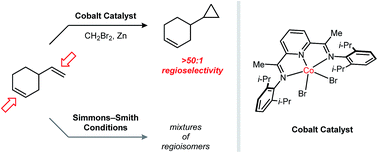
A [i−PrPDI]CoBr2 complex (PDI = pyridine-diimine) catalyzes Simmons–Smith-type reductive cyclopropanation reactions using CH2Br2 in combination with Zn. In contrast to its non-catalytic variant, the cobalt-catalyzed cyclopropanation is capable of discriminating between alkenes of similar electronic properties based on their substitution patterns: monosubstituted > 1,1-disubstituted > (Z)-1,2-disubstituted > (E)-1,2-disubstituted > trisubstituted. This property enables synthetically useful yields to be achieved for the monocyclopropanation of polyalkene substrates, including terpene derivatives and conjugated 1,3-dienes. Mechanistic studies implicate a carbenoid species containing both Co and Zn as the catalytically relevant methylene transfer agent.


 Please wait while we load your content...
Please wait while we load your content...