Ligand-enabled ortho-C–H olefination of phenylacetic amides with unactivated alkenes†
Abstract
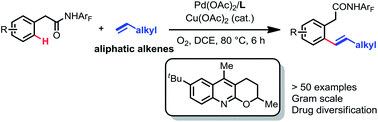
Although chelation-assisted C–H olefination has been intensely investigated, Pd(II)-catalyzed C–H olefination reactions are largely restricted to acrylates and styrenes. Here we report a quinoline-derived ligand that enables the Pd(II)-catalyzed olefination of the C(sp2)–H bond with simple aliphatic alkenes using a weakly coordinating monodentate amide auxiliary. Oxygen is used as the terminal oxidant with catalytic copper as the co-oxidant. A variety of functional groups in the aliphatic alkenes are tolerated. Upon hydrogenation, the ortho-alkylated product can be accessed. The utility of this reaction is also demonstrated by the late-stage diversification of drug molecules.
- This article is part of the themed collections: 2018 International Open Access Week Collection and 7th EuCheMS Chemistry Congress – Molecular frontiers and global challenges


 Please wait while we load your content...
Please wait while we load your content...