Closely related yet different: a borylene and its dimer are non-interconvertible but connected through reactivity†
Abstract
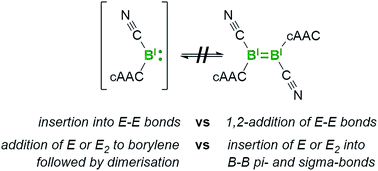
The self-stabilizing, tetrameric cyanoborylene [(cAAC)B(CN)]4 (I, cAAC = 1-(2,6-diisopropylphenyl)-3,3,5,5-tetramethylpyrrolidin-2-ylidene) and its diborene relative, [(cAAC)(CN)B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B(CN)(cAAC)] (II), both react with disulfides and diselenides to yield the corresponding cAAC-supported cyanoboron bis(chalcogenides). Furthermore, reactions of I or II with elemental sulfur and selenium in various stoichiometries provided access to a variety of cAAC-stabilized cyanoboron–chalcogen heterocycles, including a unique dithiaborirane, a diboraselenirane, 1,3-dichalcogena-2,4-diboretanes, 1,3,4-trichalcogena-2,5-diborolanes and a rare six-membered 1,2,4,5-tetrathia-3,6-diborinane. Stepwise addition reactions and solution stability studies provided insights into the mechanism of these reactions and the subtle differences in reactivity observed between I and II.
B(CN)(cAAC)] (II), both react with disulfides and diselenides to yield the corresponding cAAC-supported cyanoboron bis(chalcogenides). Furthermore, reactions of I or II with elemental sulfur and selenium in various stoichiometries provided access to a variety of cAAC-stabilized cyanoboron–chalcogen heterocycles, including a unique dithiaborirane, a diboraselenirane, 1,3-dichalcogena-2,4-diboretanes, 1,3,4-trichalcogena-2,5-diborolanes and a rare six-membered 1,2,4,5-tetrathia-3,6-diborinane. Stepwise addition reactions and solution stability studies provided insights into the mechanism of these reactions and the subtle differences in reactivity observed between I and II.
- This article is part of the themed collection: Most popular 2018-2019 main group, inorganic and organometallic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...