Beyond optical rotation: what's left is not always right in total synthesis†
Abstract
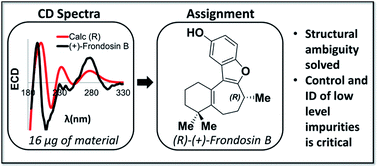
This work describes the application of vibrational (VCD) and electronic (ECD) circular dichroism spectroscopy to solve the longstanding debate around the absolute configuration of (+)-frondosin B (1). The absolute configuration of (+)-1 could confidently be assigned as (R) using these spectroscopic techniques. The discrepancy in the optical rotation (OR) values obtained in previous studies can be attributed to an undetected minor impurity (ca. 7%) that arose unexpectedly in a key step late in the synthesis. Additionally, the conditions used in the final step of the previous reports for demethylation to form the natural product proceeded with significant loss of enantiopurity. The large OR measured for the impurity at its observed level, when compared to the small rotation for the less enantiopure natural product 1, led to a measured OR value for the synthetic material that had the opposite sign of the natural product.


 Please wait while we load your content...
Please wait while we load your content...