Expedient on-resin modification of a peptide C-terminus through a benzotriazole linker†
Abstract
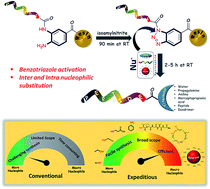
A convenient and efficient chemical toolbox was developed for the on-resin C-terminal functionalization of various peptides. By transforming resin-bound 3,4-diaminobenzoic acid species with isoamyl nitrite, the resulting resin-bound benzotriazole entity can be efficiently displaced by nucleophiles during cleavage of the peptide–resin connection in a short reaction time. The resin cleavage step allowed for the use of various nucleophiles including water, EtOH, amines, thiol, and G5 poly(amidoamino) dendrimers with yields ranging from 66% to 82% within 5 h. This method was successfully applied to prepare the elastin sequence (VPGVG)4 through on-resin ligation in 77% yield in one day and a head-to-tail cyclic peptide, sunflower trypsin inhibitor-1, in 42% yield.
- This article is part of the themed collection: Most impactful Chemical Biology articles of 2018


 Please wait while we load your content...
Please wait while we load your content...