Dual nickel and Lewis acid catalysis for cross-electrophile coupling: the allylation of aryl halides with allylic alcohols†
Abstract
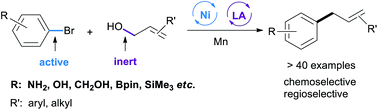
Controlling the selectivity in cross-electrophile coupling reactions is a significant challenge, particularly when one electrophile is much more reactive. We report a general and practical strategy to address this problem in the reaction between reactive and unreactive electrophiles by a combination of nickel and Lewis acid catalysis. This strategy is used for the coupling of aryl halides with allylic alcohols to form linear allylarenes selectively. The reaction tolerates a wide range of functional groups (e.g. silanes, boronates, anilines, esters, alcohols, and various heterocycles) and works with various allylic alcohols. Complementary to most current routes for the C3 allylation of an unprotected indole, this method provides access to C2 and C4–C7 allylated indoles. Preliminary mechanistic experiments reveal that the reaction might start with an aryl nickel intermediate, which then reacts with Lewis acid activated allylic alcohols in the presence of Mn.


 Please wait while we load your content...
Please wait while we load your content...