Small molecular organic nanocrystals resemble carbon nanodots in terms of their properties†
Abstract
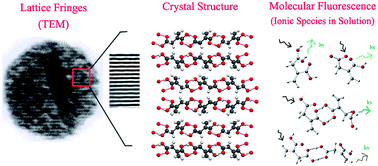
The most commonly observed phenomena in carbon nanodots (CNDs) are the strong excitation wavelength dependent multicolor fluorescence emission and the particle size distribution between 3–5 nm observed using a transmission electron microscope (TEM). However, it is not evident yet whether the emission originates from the particles observed using a TEM. In this article, we show that hydrothermal treatment of citric acid produces methylenesuccinic acid, which gives rise to hydrogen-bonded nano-assemblies with CND-like properties. While single crystal X-ray crystallography confirms the structure of methylenesuccinic acid, fluorescence correlation spectroscopy (FCS) confirms the presence of a molecular fluorophore with an average hydrodynamic diameter of ∼0.9 nm. This size is much smaller than the size of the particles observed using a TEM. We conclude that the particles observed using a TEM are the drying mediated nanocrystals of methylenesuccinic acid.
- This article is part of the themed collections: Most popular 2018-2019 physical and theoretical chemistry articles and Most popular 2018-2019 nanoscience articles


 Please wait while we load your content...
Please wait while we load your content...