Access to the β-scission rate coefficient in acrylate radical polymerization by careful scanning of pulse laser frequencies at elevated temperature†
Abstract
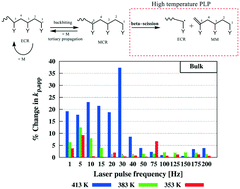
A novel method to estimate the β-scission rate coefficient (kβ) in radical polymerization of acrylates is introduced, provided that the backbiting and tertiary propagation rate coefficient have already been determined at sufficiently low temperatures at which β-scission is negligible (≪350 K). The method relies on the sensitivity of kβ upon a change of the pulse laser frequency (≪200 Hz) under isothermal pulsed laser polymerization (PLP) conditions in the temperature range between ca. 350 and 415 K, leading to a sufficient variation of the times scales of the radicals involved. These observations are not significantly influenced by macropropagation and thermal self-initiation, as respectively confirmed by in silico testing and experimental data. Solution inflection point data (e.g. solvent butyl propionate) are needed in the lower temperature range (350–410 K), whereas bulk inflection point data suffice in the higher temperature range (410–415 K). The proposed method leads to an estimated kβ value of (4.26 ± 1.8) × 102 s−1 at 413 K with bulk PLP data, suggesting a high propensity of macromonomer formation in acrylate polymerization under high temperature radical polymerization conditions, exceeding the previously suggested levels (kβ = 6 × 100–1.45 × 102 s−1).


 Please wait while we load your content...
Please wait while we load your content...