A versatile, immobilized gold catalyst for the reductive amination of aldehydes in batch and flow†
Abstract
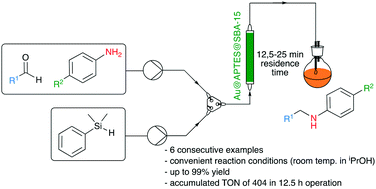
An efficient and environmentally friendly catalytic route for the reductive amination of carbonyl compounds has been designed. First, a one-pot procedure has been followed for the synthesis and incorporation of gold nanoparticles (AuNPs) into a mesoporous silica solid by using light as an abundant and clean energy source. Then, the as-prepared material has been successfully employed as a heterogeneous catalyst in the production of secondary and tertiary amines by reductive amination using Ph(Me)2SiH as the reducing agent. The endurance of the catalyst has been demonstrated in batch, and a continuous flow process allowing the safe preparation of multigram amounts of reductive amination products (accumulated TON = 434 in 18 h operation involving 6 sequential examples) has been implemented. The scope of the reaction has also been extended to the synthesis of more challenging active pharmaceutical ingredients (APIs), such as indoprofen precursors and biomass-derived products, which were obtained in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...