Direct observation of the kinetics of gas–solid reactions using in situ kinetic and spectroscopic techniques†
Abstract
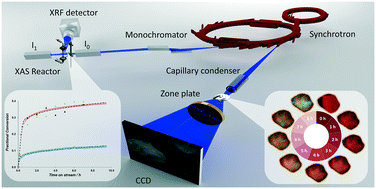
Developing fundamental insight for reactions between gas phase H2S and solid phase CuO has the potential to lead to improved materials and processes for natural gas purification. However, this insight requires detailed knowledge of the atomistic characteristics of the solid and how these characteristics influence the reaction mechanism and kinetics. Herein, we use fixed bed reactors, X-ray absorption spectroscopy, and transmission X-ray microscopy to simultaneously probe the fundamental kinetics of the reaction of CuO with H2S to form CuS, and thereby probe spatial–temporal chemical and structural changes of copper during this reaction. H2S removal reaction kinetics show similar trends in fixed bed reactors as in 10–20 μm sized particles. However, reaction fronts proceed through the entire diameter of particles heterogeneously, indicating the presence of pore diffusion resistance even at very small length scales. In addition, CuO sorbent samples with similar characteristics exhibit 3 times different sulfidation conversion with reaction rate constants that differ by a factor of 1.5. These differences in reaction kinetics and conversion indicate the critical impact of possible atomic scale differences and the formation of different copper sulfide products.


 Please wait while we load your content...
Please wait while we load your content...