The interplay between ceria particle size, reducibility, and ethanol oxidation activity of ceria-supported gold catalysts†
Abstract
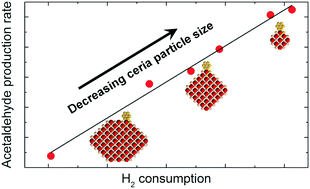
The structure of a support material can have profound impacts on the behavior of a catalyst, altering the activity and selectivity of chemical reactions. In this article, we investigate the influence of the support material's structure on the activity of Au/CeO2 catalysts for selective oxidation of ethanol in a fixed-bed flow reactor. By doping the ceria support with Al, La, and Zr during synthesis and by altering the temperature of pretreatment in air after synthesis, ceria particles varying in size between 3 nm and 22 nm were prepared. The smaller ceria particles exhibited higher oxygen storage capacities as determined by temperature programmed reduction testing and resulted in more active catalysts for ethanol oxidation. We note a linear correlation between oxygen storage capacity and catalytic activity for ethanol oxidation.


 Please wait while we load your content...
Please wait while we load your content...