Proton conductive ionic liquid crystalline poly(ethyleneimine) polymers functionalized with oxadiazole†
Abstract
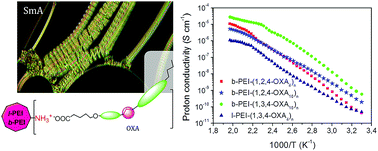
Two novel series of ionic liquid crystal polymers that display proton conductive properties are presented here. These materials are based on linear (l-PEI) or branched (b-PEI) poly(ethyleneimine) functionalized with unsymmetrical oxadiazole carboxylic acids derived from 1,3,4-oxadiazole (1,3,4-OXAm) or 1,2,4-oxadiazole (1,2,4-OXAm). The subscript “m” indicates the length of the spacer between the rigid moiety and the carboxyl group, namely m = 4 and 10. The occurrence of proton transfer from the carboxylic acid to the amine groups was confirmed by FTIR and NMR measurements. The liquid crystalline properties were investigated by differential scanning calorimetry (DSC), polarizing optical microscopy (POM), and X-ray diffraction (XRD). All ionic complexes displayed enantiotropic smectic A mesophases and in the case of the l-PEI derivatives a nematic phase was also observed at high temperatures. All investigated derivatives presented good proton conductivity values as determined by electrochemical impedance spectroscopy (EIS). Therefore, these ionic LC hyperbranched polymers represent an effective approach for the preparation of proton-transporting polymeric materials with potential applications in electrochemical devices.


 Please wait while we load your content...
Please wait while we load your content...