A green sorbent for CO2 capture: α-cyclodextrin-based carbonate in DMSO solution†
Abstract
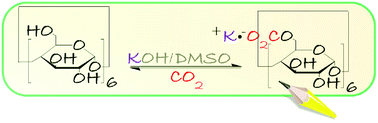
Cyclodextrin (α-CD)/KOH pellet dissolved in DMSO was utilized to capture CO2. KOH has a dual function of enhancing the nucleophilicity of the hydroxyl groups on the α-CD rims and acting as a desiccant. 13C NMR spectroscopy provided evidence for the chemisorption of CO2 through the formation of organic carbonate (RO-CO2−·K+). This was supported by the spectral changes obtained using ex situ ATR-FTIR spectroscopy upon bubbling CO2. Activation of α-CD with NaH or bubbling with 13CO2 verified that chemisorption occurred solely via RO-CO2−·K+ rather than inorganic bicarbonate. Volumetric gas uptake demonstrated a sorption capacity of 21.3 wt% (4.84 mmol g−1). To the best of our knowledge, this is the highest chemisorption value reported to date for CD-based sorbents. DFT calculations of the Gibbs free energies indicated that the formation of RO-CO2−·K+ was more favoured at the primary carbinol rather than its secondary counterpart.


 Please wait while we load your content...
Please wait while we load your content...