Design and controllable synthesis of ethylenediamine-grafted ion imprinted magnetic polymers for highly selective adsorption to perchlorate†‡
Abstract
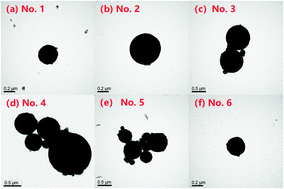
A series of ethylenediamine-grafted ion imprinted magnetic polymers (Fe3O4@IIPs) were synthesized via ultrasonic assisted suspension polymerization with perchlorate (ClO4−) as an ion imprinting template. They were characterized by XRD, EA, VSM, FTIR and XPS and applied as adsorbents for ClO4− removal from aqueous solutions. The effects of the usage amount of crosslinking agent divinylbenzene (DVB) used for preparation on the structure and the adsorptive performance of Fe3O4@IIPs were investigated. The results show that the Fe3O4@IIPs have an average size of 200–800 nm, which increases with the increase of the amount of DVB from 0 to 2 g during the preparation process. The saturation magnetization intensities are at 35.6–42.8 emu g−1, which decrease with the increase of the usage amount of DVB. The addition of DVB is beneficial to the formation and stability of the ion imprinted cavity of Fe3O4@IIPs. The effects of the solution pH value, initial concentration of ClO4−, and adsorption time on the adsorption properties of ClO4− in aqueous solutions were investigated. The results show that the adsorption capability is affected significantly by solution pH value and reaches the maximum adsorption capacity at pH 3.0. The best adsorption capacity and selectivity of Fe3O4@IIPs to ClO4− can be obtained when the usage amount of DVB is at 0.5 g for synthesis. The adsorption mechanisms might include both ion exchange and electrostatic interaction. The isothermal adsorption curves mainly obey the Langmuir model with the theoretical maximum adsorption capacities (qm,c) at 76.92–111.1 mg g−1 and the experimental maximum adsorption capacities (qm,e) at 75.7–108.9 mg g−1, respectively, which are much higher than those of the non-ion imprinted material (Fe3O4@NIP, qm,NIP: qm,c at 60.61 mg g−1 and qm,e at 59.0 mg g−1). The adsorption kinetic studies show that the adsorption processes reach equilibrium within 10 min and the kinetic data are well fitted to the pseudo-second-order model. There is almost no interference by the coexisting anions for the selective adsorption of ClO4−, with a imprinting factor (α) at 1.8, and selectivity factor (β) larger than 5.9 for several kinds of common co-existing anions, respectively. The Fe3O4@IIPs are ideal candidates for removal of ClO4− from aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...