Direct β-selectivity of α,β-unsaturated γ-butyrolactam for asymmetric conjugate additions in an organocatalytic manner†
Abstract
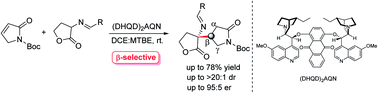
The β-selective asymmetric addition of γ-butyrolactam with cyclic imino esters catalyzed by a bifunctional chiral tertiary amine has been developed, which provides an efficient access to optically active β-position functionalized pyrrolidin-2-one derivatives in both high yield and enantioselectivity (up to 78% yield and 95 : 5 er). This is the first catalytic method to access chiral β-functionalized pyrrolidin-2-one via a direct organocatalytic approach.


 Please wait while we load your content...
Please wait while we load your content...