In situ synchrotron XAS study of the decomposition kinetics of ZDDP triboreactive interfaces
Abstract
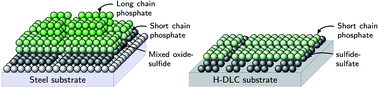
One of the major obstacles in replacing the widely used zinc dialkyldithiophosphate (ZDDP) antiwear additive with a more environmentally friendly one is the difficulty of time-resolving the surface species resulting from its decomposition mechanism under high contact pressure and temperature. To tackle this issue, a newly developed miniature pin-on-disc tribotester was coupled with synchrotron X-ray absorption spectroscopy (XAS) to perform in situ tribological tests while examining the composition of the formed triboreactive films. The results showed that in the case of bare steel surfaces the initial decomposition products are mainly zinc sulfate species, which with further shearing and heating are reduced to zinc sulfide mixed with metal oxides. The mixed base layer seems to enhance the tenacity of the subsequently formed zinc phosphate layers composing the main bulk of the protective triboreactive film. This base layer was not observed in the case of coated substrates with hydrogenated diamond-like carbon (a-C:H DLC) coating, which results in the formation of less durable films of small volume barely covering the contacting surfaces and readily removed by shear. Comprehensive decomposition pathways and kinetics for the ZDDP triboreactive films are proposed, which enable the control and modification of the ZDDP triboreactive films.


 Please wait while we load your content...
Please wait while we load your content...