Characterization and electrochemical properties of iron-doped tetrahedral amorphous carbon (ta-C) thin films†
Abstract
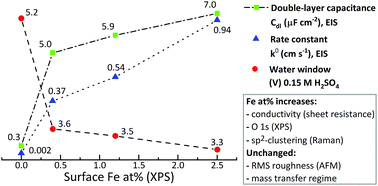
Iron-doped tetrahedral amorphous carbon thin films (Fe/ta-C) were deposited with varying iron content using a pulsed filtered cathodic vacuum arc system (p-FCVA). The aim of this study was to understand effects of iron on both the physical and electrochemical properties of the otherwise inert sp3-rich ta-C matrix. As indicated by X-ray photoelectron spectroscopy (XPS), even ∼0.4 at% surface iron had a profound electrochemical impact on both the potential window of ta-C in H2SO4 and KOH, as well as pseudocapacitance. It also substantially enhanced the electron transport and re-enabled facile outer sphere redox reaction kinetics in comparison to un-doped ta-C, as measured with electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) using outer-sphere probes Ru(NH3)6, IrCl6, and FcMeOH. These increases in surface iron loading were linked to increased surface oxygen content and iron oxides. Unlike few other metals, an iron content even up to 10 at% was not found to result in the formation of sp2-rich amorphous carbon films as investigated by Raman spectroscopy. Atomic force microscopy (AFM) and transmission electron microscopy (TEM) investigations found all films to be amorphous and ultrasmooth with Rq values always in the range of 0.1–0.2 nm. As even very small amounts of Fe were shown to dominate the electrochemistry of ta-C, implications of this study are very useful e.g. in carbon nanostructure synthesis, where irregular traces of iron can be readily incorporated into the final structures.


 Please wait while we load your content...
Please wait while we load your content...