Accelerated aging and degradation mechanism of LiFePO4/graphite batteries cycled at high discharge rates†
Abstract
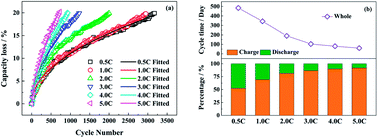
The effects of discharge rates (0.5C, 1.0C, 2.0C, 3.0C, 4.0C and 5.0C) on the aging of LiFePO4/graphite full cells are researched by disassembling the fresh and aged full cells. The capacity degradation mechanism is analyzed via electrochemical performance, surface morphologies and compositions, and the structure of the anode and cathode electrodes. The capacity fade is accelerated with increasing discharge rates. The irreversible loss of active lithium due to the generation of an SEI film is the primary aging factor for the full cells cycled at low discharge rates. However, when the discharge rate is greater than or equal to 4.0C, the performance degradation of the LiFePO4 electrode is distinct due to structure decay, which is caused by quick and repeated intercalation of lithium ions and elevated temperature during discharging. In addition, the SEI film on the anode tends to be unstable after the rapid extraction of lithium ions at high discharge rates, and this enhances the loss of active lithium. Therefore, it is indicated that the degradation mechanism is changed for the full cells aged at 4.0C and 5.0C. Besides, the high discharge rate also increases the internal resistance of the full cell, which is detrimental to high rate discharge performance.


 Please wait while we load your content...
Please wait while we load your content...