Synthesis of novel cyclopeptides containing heterocyclic skeletons†
Abstract
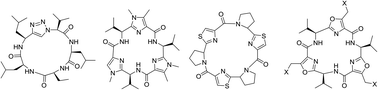
Cyclopeptides can be considered as naturally biologically active compounds. Over the last several decades, many attempts have been made to synthesize complex naturally occurring cyclopeptides, and great progress has been achieved to advance the field of total synthesis. Moreover, cyclopeptides containing heterocyclic skeletons have been recently developed into powerful reactions and approaches. This review aims to highlight recent advances in the synthesis of cyclopeptides containing heterocyclic skeletons such as triazole, oxazole, thiazole, and tetrazole.


 Please wait while we load your content...
Please wait while we load your content...