Heterocorroles: corrole analogues containing heteroatom(s) in the core or at a meso-position
Abstract
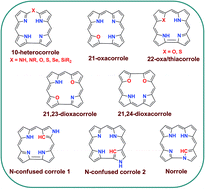
Corroles are 18 π aromatic macrocyclic systems having one direct pyrrole–pyrrole linkage leading to a contracted cavity compared to porphyrins. Corroles exhibit contrasting coordination chemistry and properties compared to porphyrins. Structural modification of corroles by introducing a heteroatom in their aromatic conjugation circuit i.e., either in the core or at a meso position leads to a new class of corrinoids called heterocorroles. The core modification strategy includes replacing one or two core nitrogen atom(s) with O, S or C atoms and meso-modification involves replacing the meso-carbon atom at the 10-position with NH, NR, O, S, Se or Si atoms. This review article presents an overview of the progress in heterocorrole chemistry including their syntheses, key structural aspects and properties.
- This article is part of the themed collection: Chemical Frontiers Goa


 Please wait while we load your content...
Please wait while we load your content...