ZnS coating for enhanced environmental stability and improved properties of ZnO thin films
Abstract
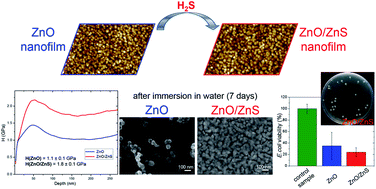
Low environmental stability of ZnO nanostructures in hydrophilic systems is a crucial factor limiting their practical applications. ZnO nanomaterials need surface passivation with different water-insoluble compounds. This study describes a one-step passivation process of polycrystalline ZnO films with ZnS as a facile method of ZnO surface coating. A simple sulfidation reaction was carried out in gas-phase H2S and it resulted in formation of a ZnS thin layer on the ZnO surface. The ZnS layer not only inhibited the ZnO dissolving process in water but additionally improved its mechanical and electrical properties. After the passivation process, ZnO/ZnS films remained stable in water for over seven days. The electrical conductivity of the ZnO films increased about 500-fold as a result of surface defect passivation and the removal of oxygen molecules which can trap free carriers. The nanohardness and Young's modulus of the samples increased about 64% and 14%, respectively after the ZnS coating formation. Nanowear tests performed using nanoindentation methods revealed reduced values of surface displacements for the ZnO/ZnS system. Moreover, both ZnO and ZnO/ZnS films showed antimicrobial properties against Escherichia coli.


 Please wait while we load your content...
Please wait while we load your content...