Transition metal triflate catalyzed conversion of alcohols, ethers and esters to olefins†
Abstract
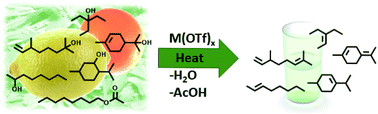
Herein, we report an efficient transition metal triflate catalyzed approach to convert biomass-based compounds, such as monoterpene alcohols, sugar alcohols, octyl acetate and tea tree oil, to their corresponding olefins in high yields. The reaction proceeds through C–O bond cleavage under solvent-free conditions, where the catalytic activity is determined by the oxophilicity and the Lewis acidity of the metal catalyst. In addition, we demonstrate how the oxygen containing functionality affects the formation of the olefins. Furthermore, the robustness of the used metal triflate catalysts, Fe(OTf)3 and Hf(OTf)4, is highlighted by their ability to convert an over 2400-fold excess of 2-octanol to octenes in high isolated yields.


 Please wait while we load your content...
Please wait while we load your content...