Nanolayer-like-shaped MgFe2O4 synthesised via a simple hydrothermal method and its catalytic effect on the hydrogen storage properties of MgH2
Abstract
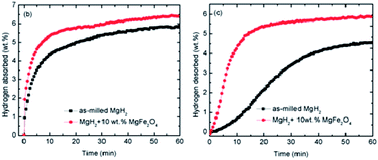
In this study, the effect of nanolayer-like-shaped MgFe2O4 that is synthesised via a simple hydrothermal method on the performance of MgH2 for hydrogen storage is studied. MgH2 + 10 wt% MgFe2O4 is prepared by using the ball milling method. The MgFe2O4-doped MgH2 sample started to release H2 at approximately 250 °C, 90 °C and 170 °C lower than the milled and pure MgH2 respectively. At 320 °C, the isothermal desorption kinetic study has shown that the doped sample has desorbed approximately 4.8 wt% H2 in 10 min while the milled MgH2 desorbed less than 1.0 wt% H2. For isothermal absorption kinetics, the doped sample can absorb approximately 5.5 wt% H2 in 10 min at 200 °C. Meanwhile, the undoped sample absorbs only 4.0 wt% H2 in the same condition. The activation energy of 10 wt% MgFe2O4-doped MgH2 composite is 99.9 kJ mol−1, which shows a reduction of 33.1 kJ mol−1 compared to the milled MgH2 (133.0 kJ mol−1). X-ray diffraction spectra display the formation of new species which are Fe and MgO after dehydrogenation, and these new species are believed to act as the real catalyst that plays a crucial role in improving the sorption performance of the MgFe2O4-doped MgH2 system by providing a synergetic catalytic effect.


 Please wait while we load your content...
Please wait while we load your content...