Formal total synthesis of histrionicotoxin alkaloids via Hg(OTf)2-catalyzed cycloisomerization and SmI2-induced ring expansion†
Abstract
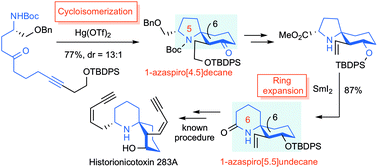
The efficient formal total synthesis of histrionicotoxin alkaloids was achieved. In this process, two key reactions were used to construct a core 1-azaspiro[5.5]undecane framework common to histrionicotoxins: a mercuric triflate (Hg(OTf)2)-catalyzed cycloisomerization of a linear substrate, which was developed in our laboratory, and a samarium iodide (SmI2)-mediated ring expansion.


 Please wait while we load your content...
Please wait while we load your content...