High-temperature thermoelectric properties of Na- and W-Doped Ca3Co4O9 system
Abstract
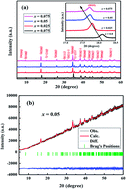
The detailed crystal structures and high temperature thermoelectric properties of polycrystalline Ca3−2xNa2xCo4−xWxO9 (0 ≤ x ≤ 0.075) samples have been investigated. Powder X-ray diffraction data show that all samples are phase pure, with no detectable traces of impurity. The diffraction peaks shift to lower angle values with increase in doping (x), which is consistent with larger ionic radii of Na+ and W6+ ions. X-ray photoelectron spectroscopy data reveal that a mixture of Co2+, Co3+ and Co4+ valence states are present in all samples. It has been observed that electrical resistivity (ρ), Seebeck coefficient (S) and thermal conductivity (κ) are all improved with dual doping of Na and W in Ca3Co4O9 system. A maximum power factor (PF) of 2.71 × 10−4 W m−1 K−2 has been obtained for x = 0.025 sample at 1000 K. The corresponding thermoelectric figure of merit (zT) for x = 0.025 sample is calculated to be 0.21 at 1000 K, which is ∼2.3 times higher than zT value of the undoped sample. These results suggest that Na and W dual doping is a promising approach for improving thermoelectric properties of Ca3Co4O9 system.


 Please wait while we load your content...
Please wait while we load your content...