Synthesis, characterization and evaluation of bulky bis(pyrazolyl)palladium complexes in Suzuki–Miyaura cross-coupling reactions†
Abstract
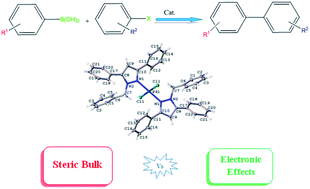
Pyrazole-containing compounds have been used in recent times as ligands to stabilize metal complexes used as pre-catalysts in cross-coupling reactions. With various substituents at various positions in the pyrazole ring, the overall electrophilic and steric properties of the metal complexes can be fine-tuned. Herein, we report the synthesis of bulky pyrazole-based ligands by condensation of methyl 4-(bromomethyl)benzoate or benzyl bromide with various substituted pyrazole compounds. These ligands were utilised in the synthesis of bis(pyrazolyl)palladium(II) complexes. The complexes' catalytic activity in Suzuki–Miyaura cross-coupling reactions was evaluated. Phenyl bearing pre-catalyst 7, at a catalyst loading of 0.33 mol%, successfully converted 98% of bromobenzene and phenylboronic acid to biphenyl in 4 h at 140 °C, while the tertiary butyl bearing pre-catalyst 8 converted up to 81% of the same substrates to biphenyl. An increase in conversion was seen for all pre-catalysts when an electron-withdrawing substituent was present on the aryl halide substrate, and the opposite was observed when the electron-withdrawing group was present on the phenyl boronic acid.


 Please wait while we load your content...
Please wait while we load your content...