Michellamines A6 and A7, and further mono- and dimeric naphthylisoquinoline alkaloids from a Congolese Ancistrocladus liana and their antiausterity activities against pancreatic cancer cells†
Abstract
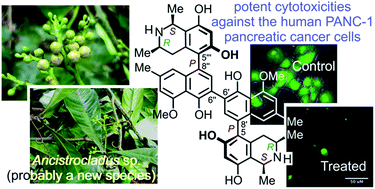
Michellamines A6 (1) and A7 (2) are the first dimers of 5,8′-coupled naphthylisoquinoline alkaloids with cis-configured stereocenters in both tetrahydroisoquinoline subunits. They were isolated from the leaves of a recently discovered, yet unidentified Congolese Ancistrocladus liana that shares some morphological characteristics with Ancistrocladus likoko. Two further new dimeric analogs, michellamines B4 (3) and B5 (4), were obtained, along with two previously likewise unknown monomers, ancistrobonsolines A1 (5) and A2 (6), which, besides one single known other example, are the only naphthyldihydroisoquinolines with an M-configured biaryl axis and R-configuration at C-3. Moreover, five compounds earlier reported from other Ancistrocladus species were identified, ancistroealaine C (7), korupensamines A (8a) and B (8b), and michellamines A2 (9) and E (10). Their complete structural elucidation succeeded due to the fruitful interplay of spectroscopic, chemical, and chiroptical methods. Chemotaxonomically, the stereostructures of the metabolites clearly delineate this Congolese Ancistrocladus liana from all known related species, showing that it might be a new taxon. Ancistrobonsolines A1 (5) and A2 (6) exhibited strong preferential cytotoxicities against human PANC-1 pancreatic cancer cells under nutrient-deprived conditions, without displaying toxicity in normal, nutrient-rich medium. Against cervical HeLa cancer cells, the dimeric alkaloids michellamines A6 (1) and E (10) displayed the highest cytotoxic activities, comparable to that of the standard agent, 5-fluorouracil. Furthermore, ancistrobonsolines A1 (5) and A2 (6) showed weak-to-moderate antiprotozoal activities.


 Please wait while we load your content...
Please wait while we load your content...