Iminoxyl radicals vs. tert-butylperoxyl radical in competitive oxidative C–O coupling with β-dicarbonyl compounds. Oxime ether formation prevails over Kharasch peroxidation†
Abstract
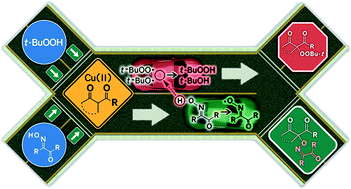
Oxidative coupling of oxime and β-dicarbonyl compounds dominates in a β-dicarbonyl compound/oxime/Cu(II)/t-BuOOH system; in the absence of oxime, oxidative coupling of t-BuOOH and a β-dicarbonyl compound (Kharasch peroxidation) takes place. The proposed conditions for oxidative coupling of oximes with dicarbonyl compounds require only catalytic amounts of copper salt and t-BuOOH serves as a terminal oxidant. The C–O coupling reaction proceeds via the formation of tert-butoxyl, tert-butylperoxyl and iminoxyl radicals. Apparently, tert-butylperoxyl radicals oxidize oxime into iminoxyl radical faster than they react with β-dicarbonyl compounds forming the Kharasch peroxidation product. Iminoxyl radicals are responsible for the formation of the target C–O coupling products; the yields are up to 77%.


 Please wait while we load your content...
Please wait while we load your content...