Synthesis and properties of soluble aromatic polyimides from novel 4,5-diazafluorene-containing dianhydride
Abstract
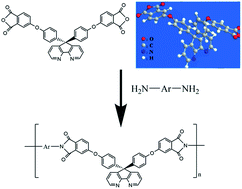
A series of organo-soluble, high glass transition temperature (Tg), and low dielectric constant aromatic polyimides containing 4,5-diazafluorene in the polymer chain were synthesized from a novel dianhydride monomer, 9,9-di[4-(3,4-dicarboxyphenoxy)phenyl]-4,5-diazafluorene dianhydride. The introduction of 4,5-diazafluorene units improved the solubility of aromatic polyimides in commonly used organic solvents, and even in dichloromethane, 1,4-dioxane and tetrahydrofuran. The obtained polyimide films also exhibited excellent thermal stability with Tg between 270 °C and 311 °C, and decomposition temperatures at 10% weight loss (T10%) between 493 °C and 552 °C. Moreover, the 4,5-diazafluorene-containing polyimides showed low dielectric constant located between 2.78 and 3.38, good mechanical properties with tensile strength between 92 and 105 MPa and elongations at break in the range of 4.49–24.8%.


 Please wait while we load your content...
Please wait while we load your content...