Auxiliary-directed etherification of sp2 C–H bonds under heterogeneous metal–organic framework catalysis: synthesis of ethenzamide†
Abstract
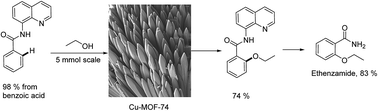
An efficient protocol for 8-aminoquinoline assisted alkoxylation and phenoxylation of sp2 C–H bonds under heterogeneous catalysis was developed. The optimal conditions employed Cu-MOF-74 (20%), K2CO3 base, pyridine ligand or dimethyl formamide solvent, and O2 oxidant at 80 °C or 100 °C for 24 hours. Cu-MOF-74 revealed remarkably higher activity when compared with other previously commonly used Cu-MOFs in cross coupling reactions, supported copper catalysts, and homogeneous copper salts. The reaction scope with respect to coupling partners included a wide range of various substrates. Interestingly, the developed conditions are applicable for the synthesis of high-profile relevant biological agents from easily accessible starting materials. Furthermore, a leaching test confirmed the reaction heterogeneity and the catalyst was reused and recycled at least 8 times with trivial degradation in activity.


 Please wait while we load your content...
Please wait while we load your content...