Polysulfides made from re-purposed waste are sustainable materials for removing iron from water†
Abstract
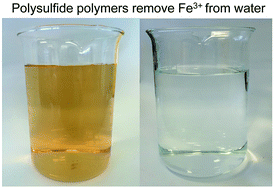
Water contaminated with Fe3+ is undesirable because it can result in discoloured plumbing fixtures, clogging, and a poor taste and aesthetic profile for drinking water. At high levels, Fe3+ can also promote the growth of unwanted bacteria, so environmental agencies and water authorities typically regulate the amount of Fe3+ in municipal water and wastewater. Here, polysulfide sorbents—prepared from elemental sulfur and unsaturated cooking oils—are used to remove Fe3+ contaminants from water. The sorbent is low-cost and sustainable, as it can be prepared entirely from waste. The preparation of this material using microwave heating and its application in iron capture are two important advances in the growing field of sulfur polymers.
- This article is part of the themed collections: Editors' collection: Supramolecular Polymers and Editors' collection: Environmental chemistry: Pollution control


 Please wait while we load your content...
Please wait while we load your content...