Comparative analysis of oxidative mechanisms of phloroglucinol and dieckol by electrochemical, spectroscopic, cellular and computational methods†
Abstract
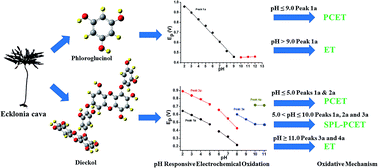
Numerous studies have been carried out on the redox activities of phenolic compounds from terrestrial plants, however, the redox pathway of phlorotannins, a type of marine algae-derived polyphenol, is far from clear. In the present study, the redox mechanisms of two phlorotannins, phloroglucinol (PL) and dieckol (DL), were comparatively scrutinized. Differential pulse voltammetry was conducted in the pH range 2.0–12.0 to determine the acid–base dissociation constant (pKa) and the number of electrons and protons involved in the redox reactions of two phlorotannins. Cyclic voltammetry was applied to obtain the heterogeneous electron transfer rate constant (k0). By means of computational calculation, UV-vis spectroscopy, and electrochemical analysis, it is proposed that PL oxidation in the whole pH range undergoes two steps which are dominated by proton-coupled electron transfer (PCET) (pH ≤ 9) and sequential proton-loss electron transfer (SPLET) mechanisms (pH > 9), respectively. In contrast, the multiple steps taking place in the DL oxidation process rely on PCET (pH ≤ 5), mixed SPLET/PCET (5 < pH ≤ 10), and electron transfer (pH > 10) mechanisms, respectively. Also, the lower proton affinity and ionization potential values of DL, which are attributed to its conjugated Cπ–O–Cπ moieties, lead to relatively higher redox activity as compared to PL in various chemical and cellular models. These findings may provide useful insights into the oxidative conversion of phlorotannins in their biological and chemical processes.


 Please wait while we load your content...
Please wait while we load your content...