Expedient synthesis of 3-hydroxypyrroles via Bu3SnH-triggered ionic 5-exo-trig-cyclization of 5-chloro-3-azamuconoate derivatives†
Abstract
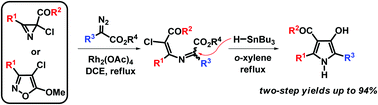
An unprecedented application of tributyltin hydride (TBTH) as a reagent for the reductive ionic 1,5-cyclization of 3-azamuconoates (3-azahexa-2,4-dienedioates) is described. A novel 1,5-exo-trig-cyclization of 5-chloro-3-azamuconoates was used as a base for the development of an effective two-step method for the preparation of 4-hydroxy-1H-pyrrole-3-carboxylic and 3-hydroxy-1H-pyrrole-2,4-dicarboxylic acids derivatives from readily available diazo esters and 2-chloro-2H-azirines or 4-chloroisoxazoles. The experimental results, as well as the DFT calculations of the pyrroles formation, are in good agreement with the ionic mechanism involving the initial hydride attack at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the azamuconoate followed by tin-promoted 1,5-cyclization.
N bond of the azamuconoate followed by tin-promoted 1,5-cyclization.


 Please wait while we load your content...
Please wait while we load your content...