Enantioselective access to multi-cyclic α-amino phosphonates via carbene-catalyzed cycloaddition reactions between enals and six-membered cyclic imines†
Abstract
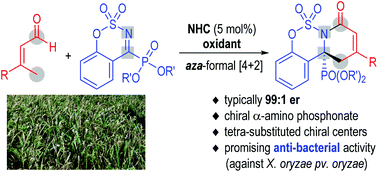
A carbene-catalyzed enantioselective cycloaddition reaction between enals and cyclic ketiminophosphonates is disclosed. α-Amino phosphonates bearing sophisticated fused heterocycles and quaternary carbons are afforded with excellent enantioselectivities. The α-amino phosphonate products from our approach exhibit encouraging anti-bacterial activities against X. oryzae pv. oryzae, the bacteria that cause serious diseases to rice and other plants.
- This article is part of the themed collection: Celebrating the 90th birthday of Professor Lu Xiyan


 Please wait while we load your content...
Please wait while we load your content...