Catalytic enantioselective synthesis of cyclopropanes featuring vicinal all-carbon quaternary stereocenters with a CH2F group; study of the influence of C–F⋯H–N interactions on reactivity†
Abstract
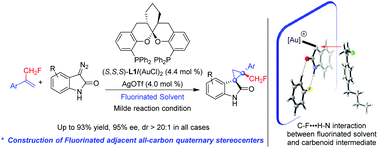
We report a highly diastereo- and enantioselective synthesis of cyclopropanes with adjacent all-carbon quaternary stereocenters featuring a monofluoromethyl (CH2F) group. While the spiroketal bisphosphine (SKP) derived chiral digold complex was identified to be a powerful catalyst for the cyclopropanation of unprotected diazooxindoles with α-CH2F styrenes, Rh2(R-DOSP)4 proved to be excellent in the corresponding reactions using aryl diazoacetate. In the cyclopropanation of diazooxindoles, dramatic rate acceleration (about twenty times) was observed when varying the reaction solvent from chlorobenzene (PhCl) to fluorobenzene (PhF), which cannot be explained by two factors that are widely used to explain the solvent effects on the olefin cyclopropanation (the dielectric constant and the coordinating ability of the solvent). Theoretical calculations suggest the formation of strong C–F⋯H–N interaction between PhF and the N–H bond of the unprotected diazooxindole derived Au(I)-carbenoid intermediate, which effectively lowers the reaction barrier. This result first demonstrated that it is possible to harness the C–F⋯H–X interaction between fluorinated solvents and the reactive intermediates to tune both the reactivity and stereoselectivity of a catalytic asymmetric reaction.


 Please wait while we load your content...
Please wait while we load your content...