Alkenylazaarenes as dipolarophiles in 1,3-dipolar cycloaddition of nitrones: regioselectivity-switchable and highly diastereoselective synthesis of multisubstituted isoxazolidines†
Abstract
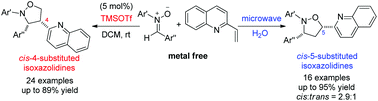
Alkenylazaarenes as a novel class of dipolarophiles for 1,3-dipolar cycloaddition (1,3-DC) with nitrones was reported. A regioselectivity-switchable synthesis of multisubstituted isoxazolidines had been developed. In the presence of TMSOTf as catalyst, 4-substituted isoxazolidines were obtained as major products with high regio- and diastereoselectivities. Nonetheless, under microwave irradiation in a sealed system with water as solvent, exclusive 5-substituted isoxazolidines were produced in good yields.
- This article is part of the themed collection: Celebrating the 90th birthday of Professor Lu Xiyan


 Please wait while we load your content...
Please wait while we load your content...