Base-promoted C–C bond cleavage for the synthesis of 2,3,4-trisubstituted pyrroles from N-propargyl β-enaminones†
Abstract
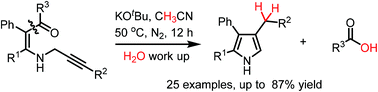
A base-promoted selective cleavage of an unstrained C(CO)–C(sp2) bond of N-propargyl β-enaminones under transition metal- and oxidant-free conditions has been established. This method allows the formation of a new C(sp2)–C(sp2) bond for the synthesis of 2,3,4-trisubstituted pyrroles under mild reaction conditions with broad functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...