Palladium-catalyzed denitrogenative cycloadditions and alkenylations of benzotriazoles with alkynes†
Abstract
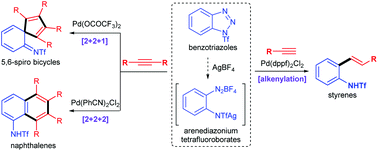
The palladium-catalyzed denitrogenative formal [2 + 2 + 1]- and [2 + 2 + 2]-cycloadditions of benzotriazoles with internal alkynes have been achieved under different reaction conditions, which enable the efficient access of highly substituted 5,6-spiro bicycles and naphthalenes, respectively. In addition, the palladium-catalyzed denitrogenative alkenylations of benzotriazoles with terminal alkynes are also reported, which offers an efficient method to access ortho-amino styrenes. The present work shows that benzotriazoles could serve as capable precursors for the divergent syntheses of various valuable scaffolds.
- This article is part of the themed collection: Celebrating the 90th birthday of Professor Lu Xiyan


 Please wait while we load your content...
Please wait while we load your content...