Asymmetric Diels–Alder cycloadditions of benzofulvene-based 2,4-dienals via trienamine activation†
Abstract
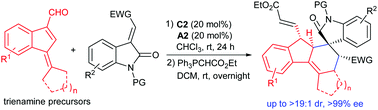
A new type of 2,4-dienal with α-substitutions and featuring a benzofulvene skeleton has been designed and utilised in asymmetric Diels–Alder cycloaddition reactions with 3-olefinic oxindoles under the trienamine catalysis of a chiral secondary amine. An array of chiral polyhydrofluorene frameworks with complex molecular structures are constructed efficiently, generally in fair to high yields with good diastereoselectivity and excellent enantioselectivity.
- This article is part of the themed collections: Celebrating the 90th birthday of Professor Lu Xiyan and Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...