Highly enantioselective desymmetrization of prochiral cyclic α,α-dicyanoalkenes via the direct vinylogous Michael/cyclization domino reaction†
Abstract
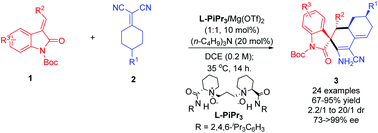
The catalytic desymmetric direct vinylogous Michael/cyclization domino reaction of 2-(4-substituted-cyclohexylidene) malononitriles with N-protected methyleneindolinones was realized by a combination of a chiral N,N′-dioxide/Mg(II) complex and an organic base. It provided efficient access to spiroindolinones bearing three adjacent stereogenic centers and a tertiary carbon center at remote sites in high yields, and with excellent dr and ee values.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...