Application of organocatalysis in bioorganometallic chemistry: asymmetric synthesis of multifunctionalized spirocyclic pyrazolone–ferrocene hybrids as novel RalA inhibitors†
Abstract
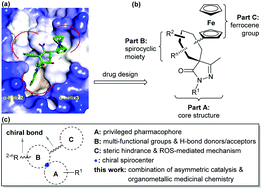
We have designed and synthesized a collection of chiral spirocyclic pyrazolone–ferrocene organometallic hybrids bearing multiple stereocenters and functional groups via organocatalysis. Compound 5b in this library displayed potent RalA inhibition, and it led to accumulation of reactive oxygen species and inhibited proliferation of pancreatic cancer cells. Molecular docking studies of 5b onto the RalA allosteric site suggest that it binds similarly to a C3 exoenzyme substrate peptide. This an efficient application of asymmetric organocatalysis in organometallic medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...