Reductive ortho C–H cyanoalkylation of aryl(heteroaryl) sulfoxides: a general approach to α-aryl(heteroaryl) nitriles†
Abstract
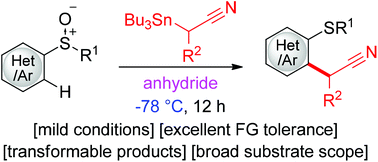
A general protocol for the synthesis of α-aryl- and heteroaryl nitriles has been developed. Specifically, the simple treatment of a mixture of aryl(heteroaryl) sulfoxides and α-stannyl nitriles with an anhydride produces a diverse array of α-aryl(heteroaryl) nitriles. Notable features of this reaction include its remarkably low reaction temperature (−78 °C), superior functional group compatibility, and highly transformable products. A DFT mechanistic study indicates that a counter anion (OCOCF3−) extracting the SnBu3 group from a nitrilium–sulfurane adduct promotes the rapid formation of a ketenimine-sulfonium salt, which undergoes a facile dearomative Claisen-type rearrangement to form the product.


 Please wait while we load your content...
Please wait while we load your content...