Stereoselective allylic reduction via one-pot palladium-catalyzed allylic sulfonation and sulfinyl retro-ene reactions†
Abstract
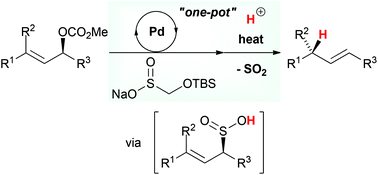
A tandem approach for the stereoselective allylic reduction has been developed based on a strategy combining the palladium-catalyzed S-allylation and the sulfinyl retro-ene reactions. In a one-pot process employing various allylic carbonates, sodium t-butyldimethylsilyloxymethanesulfinate (TBSOMS-Na), easily prepared from the commercially available reagent Rongalite™, serves as a potent nucleophile to engage in the Tsuji–Trost S-allylation reaction generating allylic sulfone products. Subsequently, in situ deprotection of the TBSOMS group reveals allylic sulfinic acids which undergo stereospecific reductive transposition via sulfur dioxide extrusion to provide alkene products.


 Please wait while we load your content...
Please wait while we load your content...